Corvus Completes Enrollment in Phase 1 Study of CPI-006 for Patients with COVID-19 and Presents Study Data at 2020 Society for Immunotherapy of Cancer (SITC) Annual Meeting
Data to be presented in a poster and an oral session “Hot Topic Symposium: COVID-19 and Cancer” at SITC
Data shows CPI-006 provided enhanced and prolonged polyclonal humoral immunity to SARS-CoV-2
Plans to initiate pivotal, randomized, double blind trial in December with results expected mid-2021
Hosting R&D Symposium webcast on
Lead investigator of the CPI-006 COVID-19 Phase 1 study,
The data presented at SITC include results from 22 patients enrolled in the Phase 1 study utilizing a cut-off date of
Results Support the Immune Enhancing Role of CPI-006 in COVID-19
- All patients had relatively low titers of anti-SARS-CoV-2 antibodies at the time of hospitalization despite having varying durations of prior COVID-19 symptoms from 1-21 days (median 5 days); all patients had a confirmed COVID-19 diagnosis by positive PCR nasal swab testing.
- All evaluable patients had prompt anti-SARS-CoV-2 antibody responses within 7 days of administration of CPI-006 at all dose levels.
- All patients recovered and were discharged from the hospital at a median of 4 (range 2-23) days.
- As of the
November 4, 2020 cut-off date, there were no drug-related toxicity or safety issues reported.
Antibody Response Results
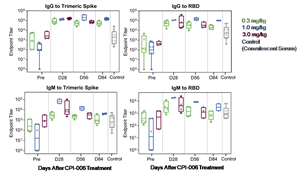
- Four of four evaluable patients that received the 0.3 mg/kg dose had sustained high titers of IgG antibodies to trimeric spike (TS) protein out to 84+ days (one patient 100+ days), without evidence of diminution of response. In these patients, IgM antibody titers peaked at 28-56 days and remained elevated out to 84+ days. Similar trends were seen in IgG and IgM antibody response to receptor binding domain (RBD).
- The geometric mean titers (GMT) for the 0.3, 1.0 and 3.0 mg/kg cohorts are shown in the charts below.
- A dose response was observed comparing the 3.0 and 1.0 mg/kg dose to the 0.3 mg/kg dose. Higher and more sustained titers of both IgG and IgM to both spike protein and RBD were seen out to 56 days when comparing the 1.0 to 0.3 mg/kg doses. The IgM responses were noteworthy for the sustained prolonged elevation.
- Antibody responses from 3.0 and 5.0 mg/kg doses appeared similar to the 1.0 mg/kg dose, but the follow up period for such doses was shorter as of the cut-off date.
- In viral neutralization assays, three of three patients developed anti-viral antibody responses out to day 56 that blocked infectivity of receptor bearing cells in a pseudovirus neutralization assay.
- Memory B cells were elevated in 6 of 6 tested patients following treatment with CPI-006. Memory T effector cells were also elevated following treatment and produced interferon-gamma and interleukin-2 in response to SARS-CoV-2 antigen consistent with antigen specific Th1 biasing.
A graphic accompanying this announcement is available at https://www.globenewswire.com/NewsRoom/AttachmentNg/7f7141d0-ea50-4293-8b5f-a4e3e8c420f6
Anti-SARS-COV-2 antibody response (IgG and IgM) to trimeric spike protein and RBD of SARS-CoV-2. Patients received a 0.3, 1.0 or 3.0 mg/kg single dose of CPI-006 and antibody titers were measured at pre-treatment and at days 28, 56 and 84. Data are shown as box and whisker plot with geometric mean and interquartile ranges. Each dot represents a patient. Also shown are titers from convalescent patients serum obtained 28-42 days after recovery from COVID-19.
Polyclonal Antibody Response
- The magnitude and diversity of the polyclonal responses were evaluated by mapping of antibody responses to the subdomains of the TS protein including the N-terminal, RBD, S1 and S2 subdomains. Polyclonality and reactivity to multiple antigenic determinants on the virus were found, which have the potential to lead to viral neutralization and elimination and to reduce the potential for escape due to emergence of mutant forms of the virus. The mapping shows:
- IgG responses were polyclonal, polyspecific and directed to all subdomains.
- IgM responses were polyclonal and directed to all subdomains but are preferentially directed to the RBD.
Preclinical Results from Humanized Mouse Studies
Vaccination studies conducted by Corvus in mice bearing human immune cells showed that administration of combinations of CPI-006 with TS protein from the SARS-CoV-2 virus resulted in an antigen specific humoral immune response to the TS protein. No humoral immune response was seen in mice receiving combinations of TS with a control antibody. The responses were specific to the immunizing TS protein as there was no reactivity to other viral proteins. These controlled studies demonstrated that the administration of CPI-006 led to the generation of antigen-specific immune response to the TS protein.
Update on CPI-006 Phase 1/1b Cancer Clinical Trial
Corvus announced that it has completed enrollment in the final cohort of the Phase 1/1b cancer clinical trial. This cohort was designed to evaluate CPI-006 in combination with ciforadenant, the Company’s A2A receptor antagonist, and pembrolizumab (triplet cohort). Other cohorts examined CPI-006 monotherapy and in combination with ciforadenant (doublet cohort). Nine patients were enrolled in the triplet cohort, including four patients with renal cell cancer (RCC). All four RCC patients remain on treatment from 2-11+ months and results, as of the
“The updated COVID-19 patient data presented at SITC continues to provide evidence regarding the unique potential immunotherapy mechanism of CPI-006, which appears to drive a robust and durable humoral response to the SARS-CoV-2 virus,” said
“In addition, we believe these data suggest CPI-006, if approved, could have broader potential to treat outpatients with COVID-19, to be used in combination with preventative vaccines to enhance and prolong immunity, and for other infectious diseases. In parallel with our pivotal study, we will be exploring these potential applications and are working tirelessly in an effort to make CPI-006 available for the treatment and prevention of COVID-19,” added
The results presented at SITC build on the initial data from the first two cohorts (0.3 mg and 1.0 mg doses) of the study that was published online at medRxiv.org in
1 Chen et
Background Information on CPI-006 for the Treatment of COVID-19
Corvus is studying an immunostimulatory humanized monoclonal antibody, designated as CPI-006, which the Company believes has demonstrated a potential new approach to immunotherapy of infectious diseases and cancer. In both in vitro and in vivo studies in cancer patients, CPI-006 has demonstrated binding to various immune cells and the inducement of a humoral adaptive immune response – B cell activation and lymphocyte trafficking leading to the production of antigen-specific immunoglobulin (IgM and IgG) antibodies. Administration of CPI-006 has also led to increased levels of memory B cells, and increased levels of both memory CD4+ and CD8+ cells, which are the cells responsible for long-term immunity. The similar production of antibodies and memory cells to pathogens such as SARS-CoV-2 may provide immediate and long-term clinical benefits for patients including shortened recovery time and improved long-term protective immunity.
To date, over 90 cancer patients have been treated with CPI-006 in the Corvus Phase 1/1b study, with dosing as high as 24 mg/kg every three weeks. The study design is evaluating CPI-006 monotherapy and in combination with ciforadenant and combination with pembrolizumab. Another cohort of the study is evaluating the triplet of CPI-006, ciforadenant and pembrolizumab. As of the
R&D Symposium Conference Call, Webcast and Presentation Slides
Corvus will host an R&D Symposium on
Tullia C. Bruno , PhD, Assistant Professor,Department of Immunology atUPMC Hillman Cancer Center .Dr. Bruno will provide an overview of B cell biology and antibodies.Gerard J. Criner , M.D., Chair and Professor, lead investigator of the CPI-006 COVID-19 Phase 1 study, and Chair and Professor, Thoracic Medicine and Surgery at theLewis Katz School of Medicine atTemple University .Dr. Criner will provide an overview of current COVID-19 therapies and results from the CPI-006 COVID-19 Phase 1 study.
Members of the Corvus team will provide an overview of the preclinical biology and data on CPI-006, plans for the CPI-006 pivotal study for patients with COVID-19, and a general pipeline update covering the Company’s cancer programs. The speakers will be available for questions and answers during the program.
The R&D Symposium conference call can be accessed by dialing 1-877-423-9813 (toll-free domestic) or 1-201-689-8573 (international) and using the conference ID 13712583. The live webcast, which will include presentation slides, may be accessed via the investor relations section of the Corvus website. A replay of the webcast will be available on Corvus' website for 90 days.
About Corvus Pharmaceuticals
About CPI-006
CPI-006 is an investigational, potent humanized monoclonal antibody that is designed to react with a specific site on CD73. In preclinical studies, it has demonstrated immunomodulatory activity resulting in activation of lymphocytes, induction of antibody production from B cells and effects on lymphocyte trafficking. While there are other anti-CD73 antibodies in development for treatment of cancer, such antibodies have been reported to react with a different region of CD73 and are designed to block production of adenosine, which is not involved in the immunomodulatory processes seen with CPI-006.
About Ciforadenant
Ciforadenant (CPI-444) is an investigational small molecule, oral, checkpoint inhibitor designed to disable a tumor’s ability to subvert attack by the immune system by blocking the binding of adenosine in the tumor microenvironment to the A2A receptor. Adenosine, a metabolite of ATP (adenosine tri-phosphate), is produced within the tumor microenvironment where it may bind to the adenosine A2A receptor present on immune cells and block their activity. CD39 and CD73 are enzymes on the surface of tumor cells and immune cells. These enzymes work in concert to convert ATP to adenosine.
Adenosine Gene Signature
The adenosine gene signature is a biomarker that reflects adenosine induced immunosuppression in the tumor. These genes express chemokines that recruit myeloid cells including immunosuppressive tumor associated CD68+ myeloid cells, which are thought to mediate resistance to anti-PD-(L)1 treatment.
Forward-Looking Statements
This press release contains forward-looking statements, including statements related to the potential safety and efficacy of ciforadenant, CPI-006, and CPI-818, the Company’s ability to develop and advance product candidates into and successfully complete preclinical studies and clinical trials, including the Company’s Phase 1/1b clinical trial of CPI-006 for certain cancers, as well as the Company’s Phase 1 trial of CPI-006 for COVID-19, the timing of the availability and announcement of clinical data and certain other product development milestones, and the sufficiency of the Company’s cash resources. All statements other than statements of historical fact contained in this press release are forward-looking statements. These statements often include words such as “believe,” “expect,” “anticipate,” “intend,” “plan,” “estimate,” “seek,” “will,” “may” or similar expressions. Forward-looking statements are subject to a number of risks and uncertainties, many of which involve factors or circumstances that are beyond the Company’s control. The Company’s actual results could differ materially from those stated or implied in forward-looking statements due to a number of factors, including but not limited to, risks detailed in the Company’s Quarterly Report on Form 10-Q for the quarter ended
INVESTOR CONTACT:
Chief Financial Officer
+1-650-900-4522
llea@corvuspharma.com
MEDIA CONTACT:
W2O pure
+1-949-903-4750
sseapy@purecommunications.com
Source: Corvus Pharmaceuticals, Inc.