Corvus Pharmaceuticals Provides Business Update and Reports Fourth Quarter and Full Year 2022 Financial Results
New CPI-818 Predictive Biomarker Enables Selection of Lymphoma Patients Most Likely to Benefit from Treatment
Conference Call Today at
“Heading into 2023, we are building momentum for CPI-818, our ITK inhibitor, which we believe is well positioned to provide a platform opportunity across cancer and immune diseases,” said
Business Update and Strategy
Prioritized Program: CPI-818 (selective ITK inhibitor)
CPI-818 for T Cell Lymphoma
- CPI-818 Phase 1/1b clinical trial results presented at the 64th
American Society of Hematology (ASH) Annual Meeting & Exposition inDecember 2022 provided clinical data and in vivo evidence supporting its ongoing development as a therapy for T cell lymphoma and its potential in autoimmune and allergic diseases. Key data from the presentation include:- As of
September 2, 2022 , there were 1 complete response (CR), 1 nodal CR and 2 partial responses (PR) in 11 evaluable patients in the 200 mg twice per day cohort (identified optimal dose). An additional PR was seen in a patient receiving the 600 mg twice per day dose. No dose limiting toxicities were observed in 43 patients enrolled across four dosing cohorts, and a maximally tolerated dose was not reached at doses as high as 600 mg twice per day. - The 200 mg dose was shown to induce Th1 skewing and both Th2 and Th17 blockade based on findings in peripheral blood samples from several patients and in vitro data demonstrated that it did so in a dose-dependent manner that supported the selection of the 200 mg twice per day optimum dose. The findings of the human and preclinical studies suggest that CPI-818 enhances anti-tumor immunity representing a potentially novel approach to immunotherapy.
- As of
- Enrollment in the 200 mg cohort has accelerated and is ongoing. As of
February 23, 2023 , 20 patients were enrolled, including 13 evaluable for tumor response. There have been 1 CR of 24 months duration, 1 equivocal CR awaiting confirmatory PET scan of 13+ months duration (a previous PR), 1 nodal CR of 21 months duration and 1 PR of 7 months duration. Ten patients continue on therapy, including seven that have not yet been evaluated for tumor response. The swimmer and waterfall tumor plots for these patients are shown below. - New CPI-818 predictive biomarker: Corvus has identified a biomarker associated with response to CPI-818. CPI-818 induces a host anti-tumor cell mediated immune response that requires normal functioning T cells. Data from the 200 mg cohort in the Phase 1/1b clinical trial indicates that a minimum absolute lymphocyte count (ALC) above 900 per cubic milliliter of blood is required for tumor response and disease control. Four of eight patients with ALC above 900 have objective responses (those four patients are described above), all eight have disease control (stable disease, PR, CR) and the median progression free survival (PFS) is 28.1 months. No objective responses were seen in five patients (0 of 5) with ALC below 900 and the PFS is 2.1 months. The ALC biomarker is routinely measured, is consistent with CPI-818’s presumed mechanism of action and is present in about 70% of patients based on the Company’s experience to-date. This biomarker has been incorporated as an eligibility criterion in the ongoing Phase 1/1b clinical trial.
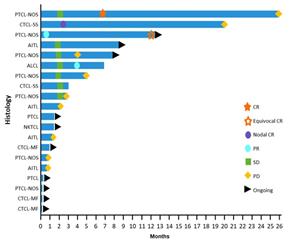
Figure 1: Swimmer Plot for Patients in the 200 mg Dose Cohort of the CPI-818 Phase 1/1b Clinical Trial for T Cell Lymphoma. The plot shows the tumor response and duration (months) for patients with various tumor histologies, which are shown on the chart and defined as follows: PTCL-NOS, peripheral T cell lymphoma not otherwise specified; CTCL-SS, cutaneous T cell lymphoma Sezary; CTCL-MF, cutaneous T cell lymphoma mycosis fungoides; AITL, angioimmunoblastic T cell lymphoma; ALCL, anaplastic T cell lymphoma and NKTCL, natural killer T cell lymphoma. The tumor response evaluation are labeled on the chart and are defined as follows: CR, complete response; equivocal CR; PR, partial response; SD, stable disease; PD, progressive disease. Arrows indicate that treatment with CPI-818 is continuing as of the
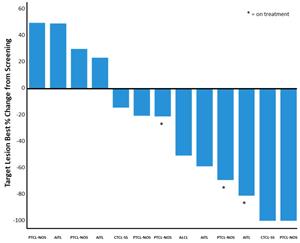
Figure 2: Waterfall Plot for Patients in the 200 mg Dose Cohort of the CPI-818 Phase 1/1b Clinical Trial for T Cell Lymphoma. The plot shows the best percent change in tumor volume in the evaluable patients from the same group shown in Figure 1.
- Corvus recently received a communication from the
U.S. Food and Drug Administration (FDA) regarding its clinical development plans for CPI-818. Based on the current enrollment rate of its ongoing Phase 1/1b clinical trial, the Company believes that the number of patients treated in this clinical trial would provide adequate safety and preliminary efficacy data to inform the design of a registration Phase 3 randomized clinical trial. As recommended by the FDA, the Company plans to meet with the FDA to discuss such a clinical trial; it is anticipated that this meeting will take place later this year.
Reprioritization of CPI -818 for Atopic Dermatitis
- Based on recent progress and data supporting the ongoing development of CPI-818 for T cell lymphoma and other cancers, Corvus has decided to delay its plans to initiate a Phase 1 clinical trial in atopic dermatitis. This decision allows the Company to conserve cash and intensify its focus on T cell lymphoma, which could include conducting a potentially registrational, randomized Phase 3 trial. While Corvus is pausing development of CPI-818 for the treatment of atopic dermatitis, the Company will continue to investigate the potential role of CPI-818 in immune diseases through its ongoing and planned preclinical research and external collaborations.
CPI-818 for HIV
- In
February 2023 , researchers from The University ofCalifornia San Francisco-Bay Area Center for AIDS Research (UCSF) presented new data at the 30th Annual Conference on Retroviruses and Opportunistic Infections demonstrating the potential of CPI-818 to reduce the need for chronic human immunodeficiency virus (HIV) therapy. The data further highlights the broad therapeutic opportunity for ITK inhibition with CPI-818. Based on this positive data, the UCSF team plans to continue studying the potential for ITK inhibition to be developed within antiproliferative and “block-and-lock” HIV cure strategies.
Partner Led Programs: Ciforadenant (adenosine 2a receptor inhibitor) and Mupadolimab (anti-CD73)
The Kidney Cancer Research Consortium (KCRC) is enrolling a Phase 1b/2 clinical trial evaluating ciforadenant as a potential first line therapy for metastatic renal cell cancer (RCC) in combination with ipilimumab (anti-CTLA-4) and nivolumab (anti-PD-1). The clinical trial is expected to enroll up to 60 patients and initial data is anticipated before the end of 2023.Angel Pharmaceuticals , Corvus’ partner inChina , is enrolling patients in a Phase 1/1b clinical trial of mupadolimab in patients with non-small cell lung cancer (NSCLC) and head and neck squamous cell cancers. In this clinical trial, patients will receive mupadolimab monotherapy or in combination with pembrolizumab.
Financial Results
As of
Research and development expenses for the three months and full year ended
The net loss for the three months ended
Conference Call Details
Corvus will host a conference call and webcast today, Tuesday, March 28, 2023, at 4:30 p.m. ET (
About Corvus Pharmaceuticals
About CPI-818
CPI-818 is an investigational small molecule drug given orally that has selectively inhibited ITK (interleukin-2-inducible T cell kinase) in preclinical studies. It was designed to block malignant T cell growth and to modulate immune responses. ITK, an enzyme, is expressed predominantly in T cells and plays a role in T cell and natural killer (NK) cell lymphomas and leukemias, as well as in normal immune function. Recent clinical data in T cell lymphomas suggests that CPI-818 has the potential to control differentiation of T helper cells and enhance immune responses to tumors. Interference with ITK signaling also can modulate immune responses to various antigens. Optimal doses of CPI-818 have been shown to affect T cell differentiation and induce the generation of Th1 helper cells while blocking the development of both Th2 and Th17 cells and production of Th2 related cytokines. Th1 T cells are required for immunity to tumors, viral infections and other infectious diseases. Th2 and Th17 helper T cells are involved in the pathogenesis of many autoimmune and allergic diseases. The immunologic effects of CPI-818 lead to what is known as Th1 skewing and is made possible by the high selectivity of CPI-818 for ITK. The Company believes the inhibition of specific molecular targets in T cells may be of therapeutic benefit for patients with T cell lymphomas, solid tumors, and in patients with autoimmune and allergic diseases. The Company is conducting a Phase 1/1b trial in patients with refractory T cell lymphomas that was designed to select the optimal dose of CPI-818 and evaluate its safety, PK, target occupancy, immunologic effects, biomarkers and efficacy. Interim data from the Phase 1/1b clinical trial of CPI-818 for T cell lymphoma demonstrated tumor responses in very advanced, refractory, difficult to treat T cell malignancies, and identified a dose that maximally affects T helper cell differentiation.
About Ciforadenant
Ciforadenant (CPI-444) is an investigational small molecule, oral, checkpoint inhibitor designed to disable a tumor’s ability to subvert attack by the immune system by blocking the binding of adenosine in the tumor microenvironment to the A2A receptor. Adenosine, a metabolite of ATP (adenosine triphosphate), is produced within the tumor microenvironment where it may bind to the adenosine A2A receptor present on immune cells and block their activity.
About Mupadolimab
Mupadolimab (CPI-006) is an investigational, potent humanized monoclonal antibody that is designed to react with a specific site on CD73. In preclinical studies, it has demonstrated immunomodulatory activity resulting in activation of lymphocytes, induction of antibody production from B cells and effects on lymphocyte trafficking. While there are other anti-CD73 antibodies and small molecules in development for treatment of cancer, such agents react with a different region of CD73. Mupadolimab is designed to react with a region of the molecule that acts to stimulate B cells and block production of immunosuppressive adenosine. Mupadolimab is being studied in combination with pembrolizumab in a Phase 1b/2 clinical trial in patients with advanced head and neck cancers and in patients with NSCLC that have failed chemotherapy and anti-PD(L)1 therapy. It is postulated that the activation of B cells will enhance immunity within the tumors of these patients, leading to improved clinical outcomes.
About
Forward-Looking Statements
This press release contains forward-looking statements, including statements related to the potential safety and efficacy of CPI-818, ciforadenant and mupadolimab; the Company’s ability and its partners’ ability, as well as the timing thereof, to develop and advance product candidates into and successfully complete preclinical studies and clinical trials, including the Company and Angel’s Phase 1/1b clinical trial of CPI-818 and the Company’s planned meeting with the FDA to discuss a registration clinical trial with CPI-818 for T cell lymphoma later this year; the design of clinical trials, including the target number of patients to be enrolled; the timing of the availability and announcement of clinical data and certain other product development milestones; the estimated amount of net cash used in operating activities for 2023 and its ability to fund operations into 2024. All statements other than statements of historical fact contained in this press release are forward-looking statements. These statements often include words such as “believe,” “expect,” “anticipate,” “intend,” “plan,” “estimate,” “seek,” “will,” “may” or similar expressions. Forward-looking statements are subject to a number of risks and uncertainties, many of which involve factors or circumstances that are beyond the Company’s control. The Company’s actual results could differ materially from those stated or implied in forward-looking statements due to a number of factors, including but not limited to, risks detailed in the Company’s Annual Report on Form 10-K for the year ended
CONDENSED CONSOLIDATED STATEMENTS OF OPERATIONS
(in thousands, except share and per share data)
| Three Months Ended |
Year Ended |
||||||||||||||
| 2022 | 2021 | 2022 | 2021 | ||||||||||||
| (unaudited) | |||||||||||||||
| Operating expenses: | |||||||||||||||
| Research and development | $ | 4,080 | $ | 4,788 | $ | 24,468 | $ | 29,115 | |||||||
| General and administrative | 1,586 | 2,022 | 8,097 | 9,515 | |||||||||||
| Total operating expenses | 5,666 | 6,810 | 32,565 | 38,630 | |||||||||||
| Loss from operations | (5,666 | ) | (6,810 | ) | (32,565 | ) | (38,630 | ) | |||||||
| Interest income and other expense, net | 318 | (8 | ) | 654 | (15 | ) | |||||||||
| Gain from sale of property and equipment | 22 | - | 22 | - | |||||||||||
| Sublease income - related party | 148 | 141 | 587 | 235 | |||||||||||
| Loss from equity method investment | (4,638 | ) | (2,559 | ) | (10,005 | ) | (4,831 | ) | |||||||
| Net loss | $ | (9,816 | ) | $ | (9,236 | ) | $ | (41,307 | ) | $ | (43,241 | ) | |||
| Net loss per share, basic and diluted | $ | (0.21 | ) | $ | (0.20 | ) | $ | (0.89 | ) | $ | (1.03 | ) | |||
| Shares used to compute net loss per share, basic and diluted | 46,553,511 | 46,551,954 | 46,553,511 | 41,854,110 | |||||||||||
CONDENSED CONSOLIDATED BALANCE SHEETS
(in thousands)
| Year ended |
|||||||
| 2022 | 2021 | ||||||
| Assets | |||||||
| Cash, cash equivalents and marketable securities | $ | 42,303 | $ | 69,451 | |||
| Operating lease right-of-use asset | 2,217 | 3,190 | |||||
| Other assets | 1,843 | 2,548 | |||||
| Investment in |
21,877 | 34,266 | |||||
| Total assets | $ | 68,240 | $ | 109,455 | |||
| Liabilities and stockholders' equity | |||||||
| Accounts payable and accrued liabilities and other liabilities | $ | 9,524 | $ | 8,646 | |||
| Operating lease liability | 2,601 | 3,647 | |||||
| Stockholders' equity | 56,115 | 97,162 | |||||
| Total liabilities and stockholders' equity | $ | 68,240 | $ | 109,455 | |||
INVESTOR CONTACT:
Chief Financial Officer
+1-650-900-4522
llea@corvuspharma.com
MEDIA CONTACT:
Real Chemistry
+1-949-903-4750
sseapy@realchemistry.com
Photos accompanying this announcement are available at:
https://www.globenewswire.com/NewsRoom/AttachmentNg/8cad1fa6-2452-4d29-9270-3ea844f7facf
https://www.globenewswire.com/NewsRoom/AttachmentNg/9fecd0a2-f763-48d1-b034-c75a997c6396

Figure 1: Swimmer Plot for Patients in the 200 mg Dose Cohort of the CPI-818 Phase 1/1b Clinical Trial for T Cell Lymphoma.
The plot shows the tumor response and duration (months) for patients with various tumor histologies, which are shown on the chart and defined as follows: PTCL-NOS, peripheral T cell lymphoma not otherwise specified; CTCL-SS, cutaneous T cell lymphoma Sezary; CTCL-MF, cutaneous T cell lymphoma mycosis fungoides; AITL, angioimmunoblastic T cell lymphoma; ALCL, anaplastic T cell lymphoma and NKTCL, natural killer T cell lymphoma. The tumor response evaluation are labeled on the chart and are defined as follows: CR, complete response; equivocal CR; PR, partial response; SD, stable disease; PD, progressive disease. Arrows indicate that treatment with CPI-818 is continuing as of the February 23, 2023 data cut-off.
Figure 2: Waterfall Plot for Patients in the 200 mg Dose Cohort of the CPI-818 Phase 1/1b Clinical Trial for T Cell Lymphoma.
The plot shows the best percent change in tumor volume in the evaluable patients from the same group shown in Figure 1.
Source: Corvus Pharmaceuticals, Inc.